Claire Brown
Sometimes, in the contorted world of food regulation, an organism that has had its DNA altered doesn’t qualify as a GMO.
This week’s news spotlighted a fascinating case in point, as USDA declared that a new non-browning mushroom, created by altering the genome of an ordinary white mushroom, Agaricus bisporus, fell outside of the agency’s authority to regulate genetically modified plants.
News coverage emphasized that this was the first CRISPR-edited organism (CRISPR being the newest generation of gene editing technology) to receive a pass from USDA. But in fact it’s just the latest in a long series of cases in which USDA has recused itself from regulating a genetically engineered plant. And the story of why that’s the case points out a gaping hole in the way this country regulates GMOs.
The urgency of the debate over genetically modified food might lead you to assume that there is a substantial body of law relating to what is allowed and what steps a product must go through as it moves to market. That’s not actually the case, and the agencies that have something to say about GMOs take their authority from laws written for other purposes. USDA’s Animal and Plant Health Inspection Service (APHIS), for instance, regulates GMO plants under a provision of the Plant Protection Act that authorizes the Secretary of Agriculture to “prohibit or restrict the importation, entry, exportation, or movement in interstate commerce of any plant, plant product [etc.] if the Secretary determines [it] is necessary to prevent the introduction . . . of a plant pest or noxious weed within the United States.”
GMOs, the argument went, are potentially plant pests because they contain genetic material from other species, but also because they had been exposed to genetically modified bacteria—which is how first-generation biotech engineers typically inserted new genes into organisms. (The logic may sound a bit off, but it’s better than the way FDA explains its authority to regulate genetically engineered animals—by classifying their modified genes as veterinary medicines.)
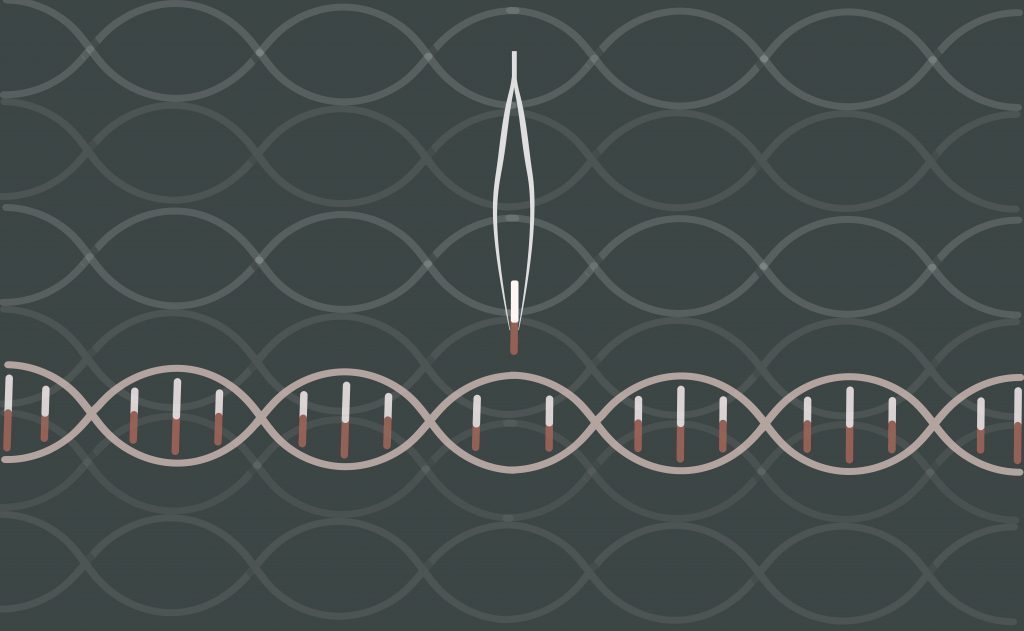
But starting about 15 years ago, scientists shifted their focus from creating transgenic organisms that contained DNA from several different species to tightly “editing” individual genes. The technology for inserting genes into cells, meanwhile shifted from using bacteria and viruses to directly injecting them using a variety of media. The result: a series of engineered organisms—a potato, several strains of soybeans—that had altered genes but no new genes from other species and no bacterial vector. And in each case USDA has declined to put them through an approval process.
The non-browning mushroom, created by Yinong Yang, a professor of plant pathology at Pennsylvania State University, follows in their footsteps, though using a technology that has been widely hailed as more powerful, easier to use, and less expensive than anything that has gone before. Using CRISPR, Yang was able to create a mushroom in which a gene for browning had been “silenced”—using a couple of months of lab time and something like $10,000 in out-of-pocket costs. His university has patented the strain, and though there are no immediate plans to market the mushroom, it seems likely that at some point someone will try. Yang has said he will consult FDA before that happens, though it is not clear that he’s required to: The agency has long taken the stand that most genetically engineered food should be treated the same as conventionally produced food.
(How can this be? Remember, the relevant laws were mostly written at a time when new strains were often produced by exposing plants to radiation and mutagenic chemicals and sorting through the unpredictable results. Genetic engineering seems pretty civilized by comparison—“enhanced interrogation” compared to the rack and the iron maiden.)
 Technology is always striving to break free of constraints, including the constraint of regulation, and it looks like bioengineered plants have officially found a way around the Plant Protection Act. That—and the sheer simplicity and low cost of the latest generation of gene editing technology—could open the door for an explosion of new food plants, some enhanced, some stripped of undesirable qualities, some perhaps restored to their original state before humans began to interfere.
Technology is always striving to break free of constraints, including the constraint of regulation, and it looks like bioengineered plants have officially found a way around the Plant Protection Act. That—and the sheer simplicity and low cost of the latest generation of gene editing technology—could open the door for an explosion of new food plants, some enhanced, some stripped of undesirable qualities, some perhaps restored to their original state before humans began to interfere.
So here’s the question: Should we let that happen?
It’s not just a question of permitting versus not permitting. In a splendid article (pay wall, but totally worth it) in Scientific American, Stephen S. Hall not only provides a fine summary of the case but raises a point that the food movement needs to pay attention to. If the U.S. moves in the direction of requiring all genetically modified plants to be approved by APHIS, it takes a process that could cost a few tens of thousands of dollars and makes it cost a few tens of millions of dollars. That means that new plant strains aren’t going to be developed by consortia of farmers or public health groups or open-source advocates. And they aren’t going to be distributed for free or at low cost. They’ll be developed by the Monsantos of the world, and they’re going to be developed to create profits.
There’s a real tension in the battle over GMOs. On the one side there’s a fear of scientific recklessness and unintended consequences. On the other, equally strong, is a fear of large corporations and their ceaseless hunger for the next dollar. In an interesting way, in this particular case, our fear of science could have its own unintended consequence: subjecting us to both reckless science and corporate greed, the worst of worlds.
 Changing the nature of plants is something we humans have done from our earliest days, and we’ve been serious about it for centuries. But it’s hard to transfer our warm feelings about a Gregor Mendel or Luther Burbank to the folks in the lab, no matter how similar their intents or the products of their work. The question to me is whether the gap we perceive can be bridged, maybe by better public understanding, but maybe by something as simple as making it possible for people with the right sorts of motivations to participate.
Changing the nature of plants is something we humans have done from our earliest days, and we’ve been serious about it for centuries. But it’s hard to transfer our warm feelings about a Gregor Mendel or Luther Burbank to the folks in the lab, no matter how similar their intents or the products of their work. The question to me is whether the gap we perceive can be bridged, maybe by better public understanding, but maybe by something as simple as making it possible for people with the right sorts of motivations to participate.
Here’s Luther Burbank on his work: “What a joy life is when you have made a close working partnership with Nature, helping her to produce for the benefit of mankind new forms, colors, and perfumes in flowers which were never known before; fruits in form, size, and flavor never before seen on this globe; and grains of enormously increased productiveness, whose fat kernels are filled with more and better nourishment, a veritable storehouse of perfect food—new food for all the world’s untold millions for all time to come.”
Now imagine those words coming from a plant pathologist in a lab coat, a mushroom in his hand. Is he the same or different? And if he’s different, what would it take to make him an ally rather than a threat? In the long run, that’s probably the most important question. Regulation, as we’ve seen this week, has a way of being left behind. Values and community may seem soft, but they’ve got a way of surviving. Talk about not turning brown.